Research
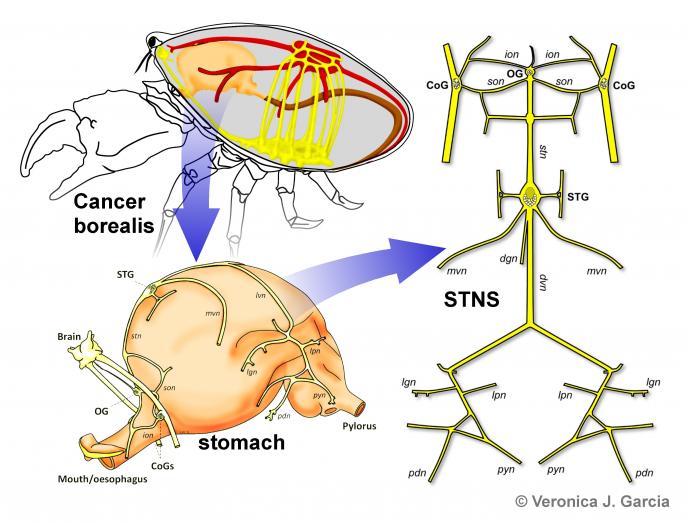
Our laboratory studies neuronal excitability and network dynamics in a small central pattern generating circuit, the crustacean stomatogastric ganglion (STG). The objective is to uncover fundamental principles that govern neural processing across animal and human nervous systems. The choice of model system comes with distinct experimental advantages, as the circuits studied contain only a small number of individually identified neurons with known synaptic connectivity. Furthermore, the STG produces very regular rhythmic activity in vitro, patterns that are straightforward to quantify and assess under different experimental conditions and in response to manipulations.
Neurons and the circuits they form produce electrical activity in a fairly complex way that cannot be understood simply on the basis of the synaptic wiring diagram. Neuronal signaling is shaped by a multitude of nonlinear dynamic properties that operate on multiple time scales. The gating properties of ion channels, short-term synaptic plasticity, neuromodulation, as well as long-term regulatory mechanisms, all contribute to activity- and time-dependent changes in excitability. We are using various electrophysiological techniques (extra- and intracellular recording, voltage clamp, current clamp, dynamic clamp, etc.), molecular biology, confocal microscopy, computational modeling and dynamical systems mathematical approaches to characterize these phenomena. We also perform cell ablations and have pioneered the use of realistic voltage waveforms in the measurement of ion channel and synaptic currents.
Mechanisms underlying network oscillations
Oscillations are ubiquitous in the central nervous system. Our research focuses on the cellular and synaptic mechanisms that underlie the generation and modification of oscillations in neural networks. Network oscillations arise as coordinated activity among populations of neurons and their abnormalities are implicated in various cognitive impairments. The role of neuronal circuits in the generation of oscillations is perhaps best studied in central pattern generators (CPGs), networks responsible for rhythmic motor activity. Given the significant role of oscillations in a multitude of behaviors and pathological conditions, it is necessary to understand how oscillations are generated in the nervous system, what controls their frequency and how oscillatory components are coordinated to produce synchronous activity. The pyloric central pattern generator (CPG) in the STG is an excellent system to address these issues. The pyloric network produces rhythms in a range of frequencies (0.5-2 Hz), controlling rhythmic contractions of striated muscles in the pylorus, a section of the foregut responsible for the filtering of masticated food. There are 11 to14 pyloric neurons located in the STG. Most of these are identified individuals with known synaptic connectivity. We study the ionic mechanisms (e.g., voltage-gated ion channels) underlying the highly nonlinear membrane behavior in these neurons, and the short-term synaptic dynamics of their connections to understand the generation of the specific electrical network activity patterns. Model networks in invertebrates have been used for decades to extract principles that were later shown to apply in mammalian networks. General principles obtained from studying the functions of synaptic dynamics in the generation and coordination of pyloric oscillations may potentially apply to other oscillatory networks that show activity-dependent changes in synaptic efficacy. Understanding these cellular and synaptic mechanisms provides important insight into the generation of self-organized oscillations of the brain, such as the multiple rhythms observed during sleep cycles or in structures involved in learning and memory formation and often affected in pathological conditions including epilepsy, depression and schizophrenia.
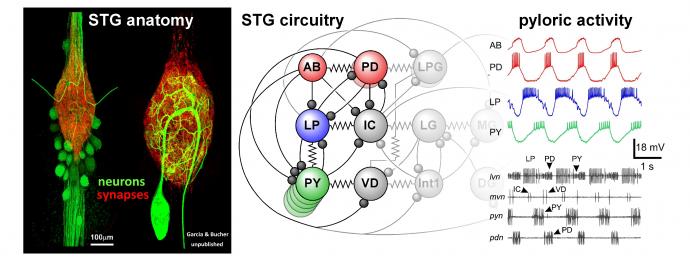
The STG contains only about 30 neurons (depending on species and inter-individual variability). Their cell bodies sit around the synaptic neuropil that is made up of fine branches from both intrinsic neurons and terminals of descending axons. The known synaptic connections consist of graded inhibitory chemical synapses and electrical coupling. The gastric mill circuit and the pyloric circuit are multiply interconnected. Shown here are the core pyloric neurons in their connectivity, and the spontaneous triphasic rhythmic activity they produce in vitro.
Neuromodulation
Neurons and the circuits they form can generate different forms of electrical activity, depending on the network state and on how they are activated. Most of this adaptability is due to neuromodulators that affect intrinsic excitability and synapses. Neuromodulators include biogenic amines like dopamine and serotonin, a vast variety of neuropeptides, but also small molecule classical transmitters that may act diffusely and/or through metabotropic receptors. The tuning of network activity is achieved through changes in the gating properties of ion channels, resulting in altered membrane behavior and excitability, and changes in the amplitude and dynamics of synaptic currents, i.e. synaptic strength and plasticity. Neuromodulators are often thought to convey global brain or behavioral states, as exemplified by the effect of serotonin on mood, the effect of norepinephrine on sleep and wakefulness, or the role of other amines or neuropeptides in eliciting specific behaviors in invertebrates. However, the idea that large regions of the brain are at any time dominated by a single neuromodulator is hard to reconcile with the complexity of actions of a multitude of neuromodulators at the neuron and circuit levels. The STNS is affected by a vast number of neuromodulators and neurohormones that produce a large degree of plasticity in the rhythmic patterns produced by these networks. Moreover, the effect of these modulators is dependent on the previous history of activity, or the state of the system. Such state-dependence produces an extra degree of plasticity. Our laboratory uses experiments to determine the cell- and neuromodulator-specific effects on excitability and synaptic connections and to measure the dynamic activity of the neurons and synapses in control conditions and in the presence of neuromodulators or while activating modulatory projection pathways.
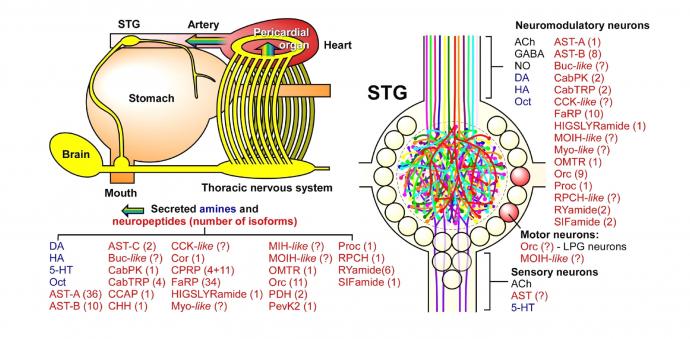
The STG is modulated by a large number of small molecule transmitters, biogenic amines, and neuropeptides. These substances are either released directly into the STG neuropil or reach the STG through the circulatory system as neurohormones. Modified from Marder and Bucher (2007) Annu Rev Physiol 69:291-316.
Long-term regulation and stability
The STNS played an important role in establishing the concept of homeostatic plasticity. There are many timescales at which neuronal and network mechanisms can be adjusted to adapt neural function to changing behavioral requirements. Considering all the activity- and neuromodulator dependent changes that circuits are subjected to in this context, there have to be mechanisms that ensure stable and robust function despite the constant flux and turnover of membrane proteins and their properties. In extreme cases, injury or other pathological conditions can be overcome by regulatory mechanisms that re-tune neuron and circuit properties to restore functional output. Findings from the STNS and other system suggest that there are complex rules underlying the homeostatic regulation of intrinsic and synaptic properties. These include a mix of both activity-dependent and activity-independent mechanisms that ultimately govern the expression levels of membrane currents. One important observation is that very similar functional phenotypes of identified neurons can be produced by hugely variable levels of individual ionic currents, as long as the expression of different types of ion channels is properly balanced. Indeed, there are cell type-specific correlations in the expression levels of different currents.
More recently, in collaboration with the Schulz Lab at Missouri University, we have identified important roles for neuromodulators in homeostatic plasticity. First, the co-regulation of intrinsic voltage-gated ion channels appears to be critically dependent on the presence of neuromodulators, as deprivation from descending neuromodulatory input destroys these relationships. Second, neuromodulator receptors, and therefore neuronal responsiveness to neuromodulators, appear to be under the control of feedback mechanisms themselves. Our data show presumably compensatory up- or down-regulation of receptors in response to manipulations of activity and modulatory environment. There is a vast literature on activity-dependent regulation of ionotropic synaptic receptors (e.g., AMPA receptors at mammalian synapses), but very little information on plasticity of metabotropic receptor expression. We are optimistic that the STG can serve as a trailblazer for our understanding of long-term regulation of neuromodulation.
Axon dynamics
Recent years have seen an increase in appreciation for the role that axonal propagation plays in neural coding. In many axons, relatively complex complements of voltage-gated ion channels and neuromodulator receptors render the temporal fidelity of spike propagation dependent on the history of activity. In consequence, activity patterns can change during conduction between spike initiation sites and synaptic terminals. Spike failures, ectopic spike initiation, and changes in interval structure have all been reported. In a series of publications, we showed that one of the stomatogastric neurons displays substantial activity-dependence of excitability and conduction delays at different time scales, including slow dynamics that outlasts slow changes found in central spike initiation. In addition, excitability is modulated by dopamine at nanomolar (hormonal) concentrations. We have identified significant parts of the underlying ionic mechanisms and modulatory signaling pathways. Biophysically realistic computational models of the axon replicate the propagation dynamics and allow inferences about the specific contributions of ionic conductances. We can also show that propagation induced changes in spike patterns, i.e. changes in the temporal neural code, can have dramatic effects on the electrical responses of postsynaptic muscle fibers.
