Publications
Current pre-prints
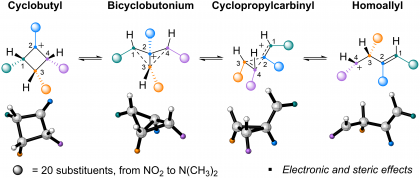
38) Larmore, S. P.; Champagne, P. A. "Substituent effects on the equilibria between cyclopropylcarbinyl, bicyclobutonium, homoallyl, and cyclobutyl cations." ChemRxiv, 2023.
Peer-reviewed articles and reviews
At NJIT
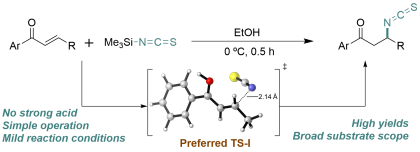
37) Li, Y.; Castañeda-Bagatella, D. M.; Kakkad, D.; Ai, Y.; Chen, H.; Champagne, P. A. "Synthetic and mechanistic study on the conjugate isothiocyanation of enones with trimethylsilyl isothiocyanate." Organic & Biomolecular Chemistry, 2023, 21, 9583-9590.
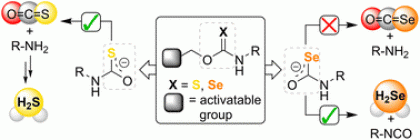
36) Newton, T. D.; Li, K.; Sharma, J.; Champagne, P. A.; Pluth, M. D. “Direct Hydrogen Selenide (H2Se) Release from Activatable Selenocarbamates” Chemical Science, 2023, 14, 7581-7588.
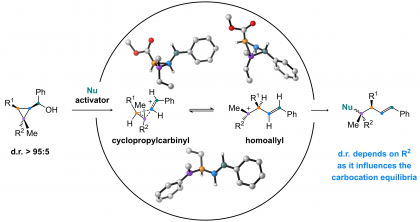
35) Larmore, S. P.; Champagne, P. A. “Cyclopropylcarbinyl-to-Homoallyl Carbocation Equilibria Influence the Stereospecificity in the Nucleophilic Substitution of Cyclopropylcarbinols.” Journal of Organic Chemistry, 2023, 88, 6947-6954.
34) Sharma, J.; Champagne, P. A. “Mechanisms of the Reaction of Elemental Sulfur and Polysulfides with Cyanide and Phosphines.” Chemistry - A European Journal, 2023, 29, e202203906.
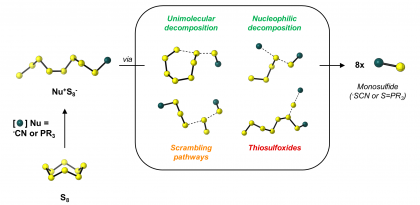
33) Ribéraud, M.; Porte, K.; Chevalier, A.; Madegard, L.; Rachet, A.; Delaunay-Moisan, A.; Vinchon, F.; Thuéry, P.; Chiappetta, G.; Champagne, P. A.; Pieters, G.; Audisio, D.; Taran, F. “Fast and Bioorthogonal Release of Isocyanates in Living Cells from Iminosydnones and Cycloalkynes.” Journal of the American Chemical Society 2023, 145, 2219-2229.
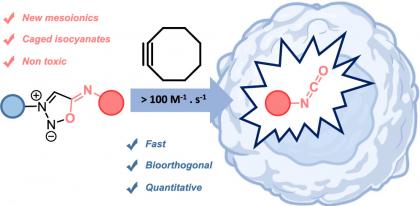
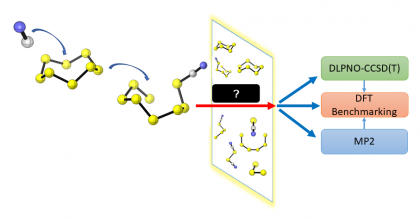
32) Sharma, J.; Champagne, P. A. “Benchmark of Density Functional Theory Methods for the Study of Organic Polysulfides.” Journal of Computational Chemistry 2022, 43, 2131-2138.
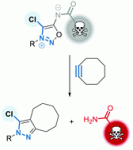
31) Feng, M.; Madegard, L.; Riomet, M.; Louis, M.; Champagne, P. A.; Pieters, G.; Audisio, D.; Taran, F. “Selective Chlorination of Iminosydnones for Fast Click and Release of Amide, Sulfonamide and Urea-Containing Drugs.” Chemical Communications, 2022, 58, 8500-8503.
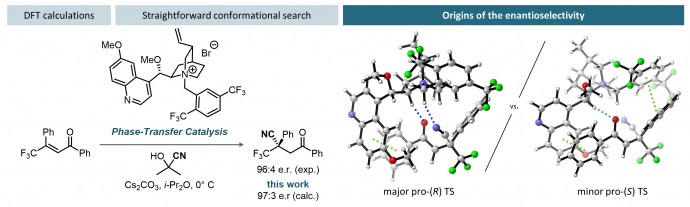
30) Buttard, F.; Champagne, P. A. “Binding Modes and Origins of Enantioselectivity in the Phase-Transfer Catalyzed Conjugate Cyanation of β-Trifluoromethylated Chalcones.” ACS Catal. 2022, 12, 8185-8194.
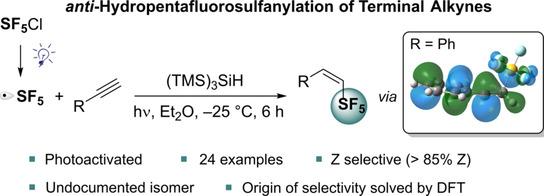
29) Birepinte, M.; Champagne, P. A.; Paquin, J.-F. "Photoinitiated anti‐Hydropentafluorosulfanylation of Terminal Alkynes." Angew. Chem. Int. Ed. 2022, 61, e202112575.
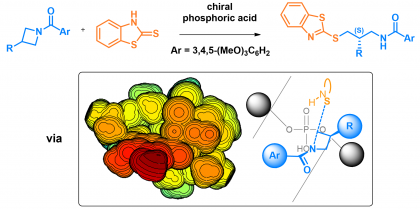
28) Champagne, P. A. "Identifying the true origins of selectivity in chiral phosphoric acid catalyzed N-acyl-azetidine desymmetrizations." Chem. Sci. 2021, 12, 15662-15672.
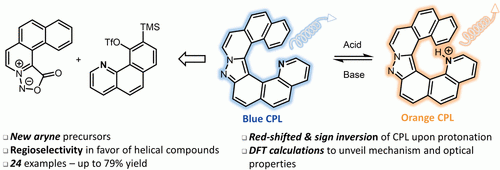
27) Yen-Pon, E.; Buttard, F.; Frédéric, L.; Thuéry, P.; Taran, F.; Pieters, G.; Champagne, P. A.; Audisio, D. “Hetero-helicenes Synthesis Through 1,3-Dipolar-Cycloaddition of Sydnones with Arynes: Synthesis, Origins of Selectivity and Application to pH Triggered Chiroptical Switch with CPL-sign Reversal” JACS Au, 2021, 1, 807-818.
26) Buttard, F.; Sharma, J.; Champagne, P. A. "Recent Advances in the Stereoselective Synthesis of Acyclic All-Carbon Tetrasubstituted Alkenes.” Chem. Commun. 2021, 57, 4071-4088.
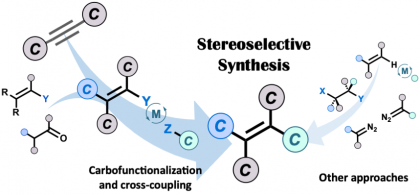
25) Nnamdi, F. U.; Diner, C.; Champagne, P. A.; Organ, M. G. “Experimental and Computational Study on the Anti‐Markovnikov Hydrofunctionalization of Olefins Using Glycine‐Extended AQ‐Auxiliaries.” Chem. Eur. J. 2021, 27, 3855-3860.
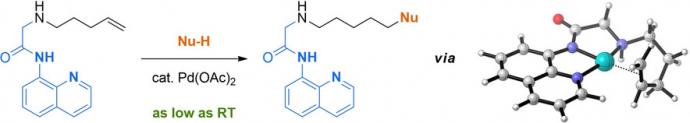
24) Liu, C.; Wang, Q.; Hivick, B. E.; Ai, Y.; Champagne, P. A.; Pan, Y.; Chen, H. "Capture of Electrochemically Generated Fleeting Carbazole Radical Cations and Elucidation of Carbazole Dimerization Mechanism by Mass Spectrometry." Anal. Chem. 2020, 92, 15291-15296.
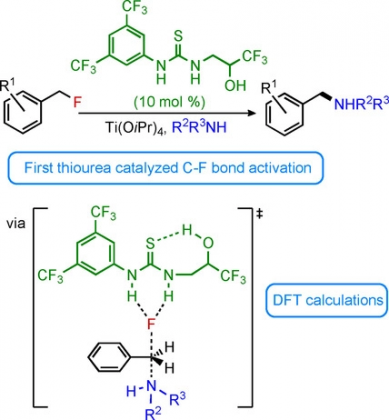
23) Houle, C.; Savoie, P. R.; Davies, C.; Jardel, D.; Champagne, P. A.; Bibal, B.; Paquin, J.-F. "Thiourea‐Catalyzed C–F Bond Activation: Amination of Benzylic Fluorides." Chem. Eur. J. 2020, 26, 10620-10625.
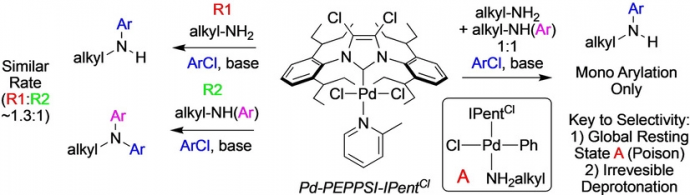
22) Lombardi, C.; Rucker, R. P.; Froese, R. D. J.; Sharif, S.; Champagne, P. A.; Organ, M. G. “Rate and Computational Studies for Pd‐NHC‐Catalyzed Amination with Primary Alkylamines and Secondary Anilines: Rationalizing Selectivity for Monoarylation versus Diarylation with NHC Ligands.” Chem. Eur. J. 2019, 25, 14223-14229.
Before NJIT
21) Sinha, N.; Champagne, P. A.; Rodriguez, M. J.; Lu, Y.; Kopach, M. E.; Mitchell, D.; Organ, M. G. “One‐Pot Sequential Kumada–Tamao–Corriu Couplings of (Hetero)Aryl Polyhalides in the Presence of Grignard‐Sensitive Functional Groups Using Pd‐PEPPSI‐IPentCl.” Chem. Eur. J. 2019, 25, 6508-6512.
20) Yen-Pon, E.; Champagne, P. A.; Plougastel, L.; Gabillet, S.; Thuéry, P.; Johnson, M.; Muller, G.; Pieters, G.; Taran, F.; Houk, K. N.; Audisio, D. “Sydnone-Based Approach to Heterohelicenes through 1,3-Dipolar-Cycloadditions.” J. Am. Chem. Soc. 2019, 141, 1435-1440.
19) Shao, Z.; Liu, W.; Tao, H.; Liu, F.; Zeng, R.; Champagne, P. A.; Cao, Y.; Houk, K. N.; Liang, Y. “Bioorthogonal release of sulfonamides and mutually orthogonal liberation of two drugs.” Chem. Commun. 2018, 54, 14089-14092.
18) Kishimoto, S.; Hara, K.; Hashimoto, H.; Hirayama, Y.; Champagne, P. A.; Houk, K. N.; Tang, Y.; Watanabe, K. “Enzymatic One-step Ring Contraction for Quinolone Biosynthesis.” Nat. Commun. 2018, 9, 2826.
17) Goh, S. S.; Champagne, P. A.; Guduguntla, S.; Kikuchi, T.; Fujita, M.; Houk, K. N.; Feringa, B. L. “Stereospecific Ring Contraction of Bromocycloheptenes Through Dyotropic Rearrangements via Non-Classical Carbocation-Anion Pairs.” J. Am. Chem. Soc. 2018, 140, 4986-4990.
16) Keddie, N. S.; Champagne, P. A.; Desroches, J.; Paquin, J.-F.; O’Hagan, D. “Stereochemical Outcomes of C–F Activation Reactions of Benzyl Fluoride.” Beilstein J. Org. Chem. 2018, 14, 106-113.
15) Simmons, B. J.; Hoffmann, M.; Champagne, P. A.; Yamakawa, K.; Picazo, E.; Houk, K. N.; Garg, N. K. “Understanding and Interrupting the Fischer Azaindolization Reaction.” J. Am. Chem. Soc. 2017, 139, 14833-14836.
14) Champagne, P. A.; Houk, K. N. “Influence of Endo- and Exocyclic Heteroatoms on Stabilities and 1,3-Dipolar Cycloaddition Reactivities of Mesoionic Azomethine Ylides and Imines.” J. Org. Chem. 2017, 82, 10980-10988.
13) Maji, R.; Champagne, P. A.; Houk, K. N.; Wheeler, S. E. “Activation Mode and Origin of Selectivity in Chiral Phosphoric Acid-Catalyzed Oxacycle Formation by Intramolecular Oxetane Desymmetrizations.” ACS Catal. 2017, 7, 7332-7339.
12) Narayanam, M. K.; Ma, G.; Champagne, P. A.; Houk, K. N.; Murphy, J. M. “Synthesis of [18F]Fluoroarenes via Nucleophilic Radiofluorination of N-Arylsydnones.” Angew. Chem. Int. Ed. 2017, 56, 13006-13010.
- Highlighted by a Synpacts article: Synlett, 2018, 29, 1131-1135.
11) Champagne, P. A.; Houk, K. N. “Origins of Selectivity and General Model for Chiral Phosphoric Acid-Catalyzed Oxetane Desymmetrizations.” J. Am. Chem. Soc. 2016, 138, 12356-12359.
10) Hemelaere, R.; Champagne, P. A.; Desroches, J.; Paquin, J.-F. “Faster Initiation in the Friedel-Crafts Reaction of Benzyl Fluorides Using Trifluoroacetic Acid as Activator.” J. Fluorine Chem. 2016, 190, 1-6.
9) Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. “Monofluorination of Organic Compounds: 10 Years of Innovation.” Chem. Rev. 2015, 115, 9073-9174. (Invited contribution)
8) Desroches, J.; Champagne, P. A.; Benhassine, Y.; Paquin, J.-F. “In Situ Activation of Benzyl Alcohols With XtalFluor-E: Formation of 1,1-Diarylmethanes and 1,1,1-Triarylmethanes Through Friedel–Crafts Benzylation.” Org. Biomol. Chem. 2015, 13, 2243-2246.
7) Champagne, P. A.; Desroches, J.; Paquin, J.-F. “Organic Fluorine as a Hydrogen-Bond Acceptor: Recent Evidence and Applications.” Synthesis 2015, 47, 306-322. (Invited contribution)
6) Champagne, P. A.; Drouin, M.; Legault, C. Y.; Audubert, C.; Paquin, J.-F. “Revised Mechanistic Explanation for the Alcohol-Promoted Amination of Benzylic Fluorides Under Highly Concentrated Conditions: Computational and Experimental Evidence on a Model Substrate.” J. Fluorine. Chem. 2015, 171, 113-119. (Invited contribution)
5) Champagne, P. A.; Benhassine, Y.; Desroches, J.; Paquin, J.-F. “Friedel-Crafts Reaction of Benzyl Fluorides: Activation of C–F Bonds as Enabled by Hydrogen-Bonding.” Angew. Chem. Int. Ed. 2014, 53, 13835-13839.
4) Champagne, P. A.; Saint-Martin, A.; Drouin, M.; Paquin, J.-F. “Triol-Promoted Activation of C–F Bonds: Amination of Benzylic Fluorides Under Highly Concentrated Conditions Mediated by 1,1,1-Tris(hydroxymethyl)propane.” Beilstein J. Org. Chem. 2013, 9, 2451-2456.
3) Champagne, P. A.; Pomarole, J.; Thérien, M.-È.; Benhassine, Y.; Beaulieu, S.; Legault, C. Y.; Paquin, J.-F. “Enabling Nucleophilic Substitution Reactions of Activated Alkyl Fluorides through Hydrogen Bonding.” Org. Lett. 2013, 15, 2210-2213.
2) Landelle, G.; Turcotte-Savard, M.-O.; Marterer, J.; Champagne, P. A.; Paquin, J.-F. “Stereocontrolled Access to Unsymmetrical 1,1-Diaryl-2-fluoroethenes.” Org. Lett. 2009, 11, 5406-5409.
1) Landelle, G.; Champagne, P. A.; Barbeau, X.; Paquin, J.-F. “Stereocontrolled Approach to Bromofluoroalkenes and Their Use for the Synthesis of Tri- and Tetra-Substituted Fluoroalkenes.” Org. Lett. 2009, 11, 681-684.
Book chapters
2) Champagne, P. A.; Drouin, M.; Paquin, J.-F. Benzylic fluorides. In Science of Synthesis Knowledge Updates, Carreira, E. M. (Ed.); Thieme: Stuttgart, 2016; Section 34.6.2, online.
1) Champagne, P. A.; Paquin, J.-F. Trifluoro(N-methylmethanaminato)sulfur. In Electronic Encyclopedia of Reagents for Organic Synthesis, Paquette, L. A. (Ed.); John Wiley & Sons Ltd.: Chichester, 2013; online.
