LC/MS
It is difficult to identify unknown compound from the UV spectrum obtained using most common in HPLC ultra violet or photodiode array detectors (PDA). The only detector capable to provide qualitative (identification) information in HPLC is mass spectrometer.
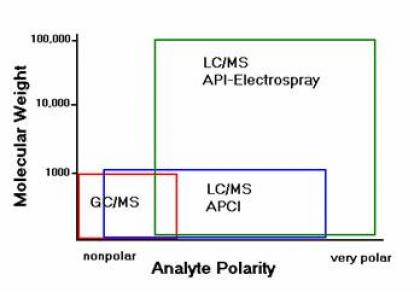
Despite the power of GC/MS, only small percent of the 9 million registered compounds can be analyzed with the GC/MS. Similar to the GC/MS, LC/MS data provide information about the molecular weight, structure, identity and quantity of non-volatile, thermally labile, or charges compounds. Good examples are phenols, polymers, explosives, dyes, and biological compounds.
The figure below is a good illustration of the choice of the appropriate analytical technique for different classes of compounds.
Agilent 6470 Triple Quadrupole LC/MS system
Agilent LC_MS 6470 Specifications.pdf ![]() Agilent 6470 Triple Quadrupole LC-MS System.pdf
Agilent 6470 Triple Quadrupole LC-MS System.pdf
- Enhanced sensitivity - detection limits up to sub-femtogram level
- Equipped with Jet Stream (5X the sensitivity of ESI) – provides greater signal and reduced noise- and APCI
- Mass range: m/z 5-3,000
- Mass Resolution: 0.7 Da
- Equipped with Diode array detector
|
Current LC/QQQ configuration and setup: USEPA Method 537: Determination of Selected Perfluorinated Alkyl Acids in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS). |
DEFINITIONS/ABBREVIATIONS
AP-spheric Pressure Electrospray Ionization:
- Ions formed by solution chemistry
- Good for thermal labile analytes
- Good for polar analytes
- Good for large molecules, such as proteins and peptides
APCI – Atmospheric Pressure Chemical Ionization:
- Ions formed by gas phase chemistry
- Good for volatile and/or thermally stable analytes
- Good for non-polar analytes
- Good for small molecules
- Mechanism:
Primary ion formation: H2O + e- ----> H2O+. + 2e-
Secondary ion formation: H2O+. + H2O ----> H3O+ + OH.
Positive analyte ion formation: H3O+. + M (analyte) ----> (M+H)+ (protonated) + H2O
Negative analyte ion formation: OH. + M (analyte) -----> (M-H)+ (deprotonated) + H2O
